Use of RNA interference to suppress gene expression of vitellogenesis-inhibiting hormone in the whiteleg shrimp, Litopenaeus vannamei
Description
The shrimp culture industry continues to expand worldwide, and now boasts a production scale of over 5 million tons per year and market volume of nearly 30 billion U.S. dollars (FAO, 2019). In commercial shrimp hatcheries, eyestalk ablation is routinely performed because this procedure removes the source of vitellogenesis-inhibiting hormone (VIH) and allows ovarian maturation to proceed. However, it is currently viewed in the aquaculture industry that it is essential to develop a technology that could replace eyestalk ablation, with the aim of making seed production more efficient and animal-friendly. In order to develop a new maturation promoting technology that can replace eyestalk ablation, RNA interference is used here to suppress VIH gene expression, and thus decrease the synthesis of endogenous VIH.
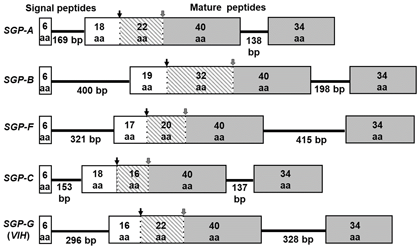
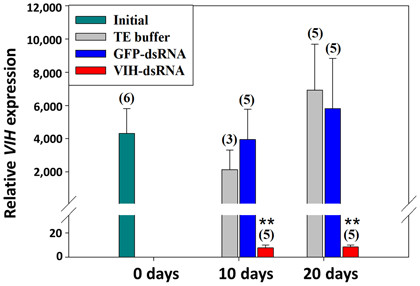
In Litopenaeus vannamei, seven peptides (sinus gland peptides: SGP-A to -G) have been previously identified from the sinus glands in the eyestalks. Among these peptides, six peptides (A, B, C, E, F, G) have been demonstrated to possess “VIH” activity; VIH suppresses the synthesis of egg yolk protein. Against this background, we cloned full-length cDNAs from the eyestalks, and also elucidated the gene structure of these five VIHs (Fig. 1). Moreover, using this genetic information, we established a quantitative real-time PCR system for multiple VIHs in L. vannamei, and the expression level of each VIH in the eyestalk was determined in relation to molting and eyestalk ablation in separate individuals. For purposes of development of new techniques for the artificial control of ovarian maturation, we utilized RNA interference to suppress VIH gene expression. Firstly, double-stranded RNA (dsRNA) for targeting the most abundant VIH gene in L. vannamei (SGP-G) was artificially synthesized. Next, it was injected into female adults (50 - 70 g body weight) at a final concentration of 3 µg per g body weight. As a result, VIH gene expression was significantly decreased both 10 and 20 days after VIH-dsRNA injection; however, there were no observed significant differences in the other groups (Fig. 2). VIH expression levels could be suppressed to less than 10% of original levels up to 20 days with only a single injection of VIH-dsRNA. Hence, using RNA interference, it has become possible to artificially suppress VIH gene expression.
Furthermore, it is possible that by simultaneously administering dsRNA complementary to not only the major VIH (SGP-G), but also to dsRNAs complementary to several other VIH genes, it may be possible to achieve even greater suppression of VIH action. In subsequent applications, we suggest that this technique may be applicable to other penaeid shrimp species that harbor similar amino acid sequences for VIH. We intend to continue this research in order to make possible the establishment of a new shrimp-friendly seed production technology and contribute to the sustainable development of the shrimp aquaculture industry.
Figure, table
-
Fig. 1. Schematic diagram of gene structure for SGPs having vitellogenesis-inhibiting hormone activity. Exons are indicated as boxes with numbers of amino acid residues (aa), and introns are indicated as bold lines with numbers of base pairs (bp). Reproduced from Kang et al., 2018 with permission from Fisheries Science.
-
Fig. 2. Suppression of SGP-G gene expression following VIH-dsRNA injection. Groups are indicated as follows. Initial: non-treatment; TE buffer: shrimp injected with TE buffer as a vehicle control; GFP-dsRNA: shrimp injected with dsRNA for green fluorescent protein (GFP); and VIH-dsRNA: shrimp injected with VIH-dsRNA. Numbers in parentheses above the bars indicate the number of individuals analyzed. Reproduced from Kang et al., 2019 with permission from Aquaculture.
- Affiliation
-
Japan International Research Center for Agricultural Sciences Fisheries Division
- Classification
-
Technical A
- Program name
- Term of research
-
FY2019 (FY2016-FY2020)
- Responsible researcher
-
Kang Bong Jung ( Fisheries Division )
ORCID ID0009-0004-9970-6923KAKEN Researcher No.: 00649022Wilder Marcy N. ( Fisheries Division )
ORCID ID0000-0003-2114-2000KAKEN Researcher No.: 70360394 - ほか
- Publication, etc.
-
FAO. 2019. FAO yearbook. Fishery and Aquaculture Statistics 2017/FAO annuaire.
(http://www.fao.org/documents/card/en/c/ca5495t)https://doi.org/10.1007/s12562-018-1212-7Kang BJ et al. (2018) Fisheries Science, 84: 649–662
https://doi.org/10.1016/j.aquaculture.2019.03.028Kang BJ et al. (2019) Aquaculture, 506: 119-126
- Japanese PDF
-
2019_D01_A4_ja.pdf876.73 KB
2019_D01_A3_ja.pdf176.77 KB
- English PDF
-
2019_D01_A4_en.pdf183.39 KB
2019_D01_A3_en.pdf244.78 KB
- Poster PDF
-
2019_D01_poster_fin.pdf320.31 KB