Identification of vitellogenesis-inhibiting hormone (VIH) in the whiteleg shrimp, Litopenaeus vannamei
Description
The Fisheries Division is conducting basic physiological research on reproductive mechanisms in the whiteleg shrimp Litopenaeus vannamei (Fig. 1), as part of a project in cooperation with the private sector to develop recirculating aquaculture systems and domestic-based seed production for this species. L. vannamei, which is native to the western hemisphere, has become an important species in shrimp culture throughout the world, but especially in Southeast Asia. To gain a better understanding of the regulatory mechanisms of vitellogenesis in this species, research was conducted to identify and characterize the biological activity of vitellogenesis-inhibiting hormone (VIH). The presence of hormones harboring molt-inhibiting and hyperglycemic activities in crude sinus gland extracts has been reported in several crustacean species. These substances are thought to harbor VIH activity as well, but this has been a difficult point to clarify.
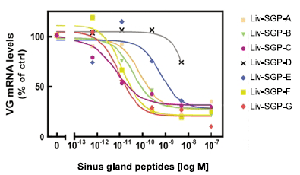
Vitellogenesis-inhibiting hormone (VIH) in Crustacea belongs to the crustacean hyperglycemic hormone (CHH) family. In this study, to characterize multiple VIH molecules in L. vannamei, seven CHH family peptides, designated as Liv-SGP-A, -B, -C, -D, -E, -F, and -G, were purified by reversed-phase high-performance liquid chromatography (RP-HPLC) and identified by N-terminal amino acid sequencing. Peptides were extracted from a total of 1,300 sinus glands and fractionated by RP-HPLC using acetonitrile containing trifluoroacetic acid (TFA) as a solvent. The dose-response effects of these peptides on vitellogenin mRNA levels were examined using in vitro incubation of ovarian fragments of the kuruma prawn, Marsupenaeus japonicus. Liv-SGP-D showed no significant inhibitory activities, while the other six peptides significantly reduced vitellogenin mRNA levels, although having differing efficacies, in the order of Liv-SGP-C, -F, -G > -A, -B > -E (Fig. 2). Liv-SGP-G was the most abundant CHH-family peptide in the sinus gland and showed strong vitellogenesis-inhibiting activity. Further structural analysis of this peptide was therefore possible. In order to determine the cysteine residues that characterize CHH-family peptides, native Liv- SGP-G was reduced, carboxymethylated, and subsequently digested with lysyl endopeptidase, resulting in two peptide fragments which were subjected to further analysis. Amino acid sequence analyses of these two peptides identified six residues at positions 7, 23, 26, 39, 43, and 52 as cysteine based on the detection of a peak of phenylthiohydantoin derivative of carboxymethylated cysteine on the HPLC chromatogram. In total, as a result of a detailed structural analysis, its complete primary structure was determined: it consists of 72 amino acid residues and possesses an amidated C-terminus.
In conclusion, six sinus gland peptides Fig. 1. Cultured Litopenaeus vannamei from a production trial with International Mariculture Technology (IMT) Co. (Photo: S. Nohara, IMT) having VIH activity were isolated from L. vannamei. The dynamics by which these six peptides are involved in the regulation of vitellogenesis in L. vannamei, however, remain unclear. To clarify this point, further experiments examining their in vivo effects and changes in their circulating levels during vitellogenesis are required. It is hoped that further work along these lines will assist in the development of technology to better control reproduction in captivity of this species.
Figure, table
-
Fig. 1. Cultured Litopenaeus vannamei from a production trial with International Mariculture Technology (IMT) Co.
(Photo: S. Nohara, IMT) -
Fig. 2. The dose-response effects of CHH-family peptides from L. vannamei on vitellogenin (VG) mRNA levels in incubated ovarian fragments.
- Affiliation
-
Japan International Research Center for Agricultural Sciences Fisheries Division
- Classification
-
Technical A
- Term of research
-
FY2006(FY2006~2010)
- Responsible researcher
-
Wilder Marcy N. ( Fisheries Division )
ORCID ID0000-0003-2114-2000KAKEN Researcher No.: 70360394OHIRA Tsuyoshi ( Fisheries Division )
Tsutsui Isao ( Fisheries Division )
KAKEN Researcher No.: 80425529KAWAZOE Ichiro ( Fisheries Division )
- ほか
- Publication, etc.
-
Tsutsui, N., Katayama, H., Ohira, T., Nagasawa, H., Wilder, M.N. and Aida, K. (2005). The effects of crustacean hyperglycemic hormone-family peptides on vitellogenin gene expression in the kuruma prawn, Marsupenaeus japonicus. General and Comparative Endocrinology, 144: 232-239.
Ohira, T., Okumura, T., Suzuki, M., Yajima, Y., Tsutsui, N., Wilder, M.N. and Nagasawa, H. (2006). Production and characterization of recombinant vitellogenesis-inhibiting hormone from the American lobster Homarus amaricanus. Peptides, 27: 1251-1258
Ohira, T., Tsutsui, N., Nagasawa, H. and Wilder, M.N. (2006). Preparation of two recombinant crustacean hyperglycemic hormone isoforms from the giant freshwater prawn Macrobrachium rosenbergii and their biological activities. Zoological Science, 23: 383-391.
Tsutsui, N., Ohira, T., Kawazoe, I., Takahashi, A. and Wilder, M.N. (2007). Purification of sinus gland peptides having vitellogenesis-inhibiting activity from the whiteleg shrimp Litopenaeus vannamei. Marine Biotechnology (In press)
- Japanese PDF
-
2006_seikajouhou_A4_ja_Part20.pdf549.29 KB