Biological nitrification inhibition of sorghum is related to the inhibition of ammonia-oxidizing archaea
Description
To increase crop production, farmers often apply high amounts of nitrogen fertilizers in agricultural lands. The resulting high nitrification activity greatly contributes to global warming due to the release of the potent nitrous oxide (N2O) gas. Furthermore, this practice not only causes environmental water pollution due to leakage of nitrate nitrogen, but also lower use efficiency of the fertilizer nitrogen and reduced crop yields. Biological nitrification inhibition (BNI), a crop-mediated complex molecular mechanism in which the crop suppresses soil nitrification by itself, is gaining much attention as a suitable technique to mitigate the above problems. Here we look at sorghum, the world’s fifth-ranked cereal in terms of production area. Sorghum secretes sorgoleone, a compound that has shown BNI ability, from its roots.
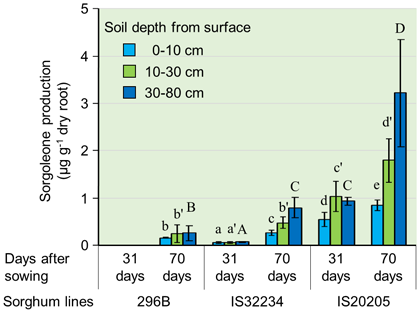
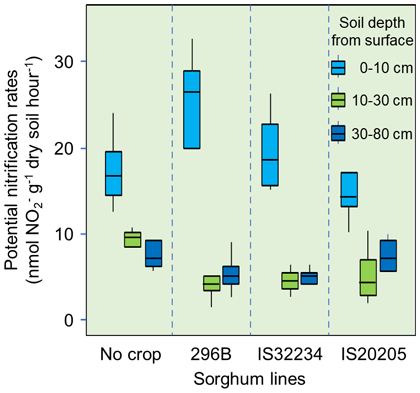
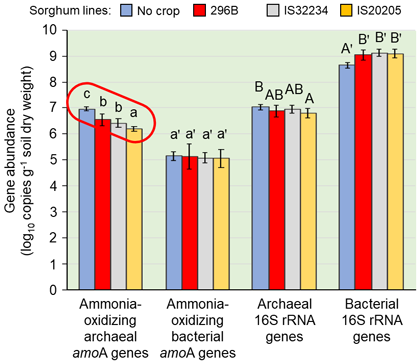
In this study, we clarified the relationships between the amount and the location of sorgoleone secretion from the plant’s roots, and the rhizosphere soil microbial communities through a pipe cultivation test (Fig. 1). 296B shows the least sorgoleone secretion, followed by IS32234 then IS20205 (Fig. 2). The secretion increases towards deeper layers at the newer root zone. The application of 120 kg ha-1 nitrogen as ammonium sulfate solution to the topsoil, greatly enhances the nitrification activity in the 0-10 cm soil layer, which remained low in the deeper layers (Fig. 3). In the 0-10 cm soil layer, 296B showed the highest nitrification activity, followed by IS32234 and IS20205, which opposes with the sorgoleone secretion. These results indicate that sorgoleone plays a substantial role in sorghum’s ability to exert BNI. The number of ammonia-oxidizing bacteria (AOB) and archaea (AOA) in the 0-10 cm soil layer was determined by qPCR, targeting the ammonia monooxygenase (amoA) gene. The number of AOA was inversely related to the amount of sorgoleone and was proportional to the nitrification activity. However, we observed no change in the number of AOB (Fig. 4). This shows that the nitrification in sorghum rhizosphere soil is related to the effect of sorgoleone secreted from the roots of sorghum, which suppresses AOA among the microorganisms harboring the amoA gene. The study also shows the contribution of other factors, such as soil pH, water content, organic and inorganic nitrogen content, to the ability of sorghum to exert BNI.
These findings demonstrate the great influence of the suppression of AOA on sorghum BNI. In addition, the ease of handling of root and soil samples by soil depth, makes this pipe cultivation test useful for BNI research of other plants.
Figure, table
-
Fig. 1. Sorghum pipe (12 cm x 1 m) cultivation test in greenhouse at 31 days after seeding (a), and soil column removed from pipe at the first soil and plant root sampling (b)
-
Fig. 2. Dynamics of sorgoleone secretion from roots of sorghum along the soil profile under nitrogen fertilizer application (120 kg ha-1)
Bars in the figure indicate standard deviations. The same type of letter (x, x′, X) compares the differences of sorgoleone secretion between sorghum lines by soil depth, and values with the same letter are not significantly different at p<0.05. -
Fig. 3. Nitrification activity in bulk (no crop) and sorghum rhizosphere soils along the soil profile at 70 days after seeding, under nitrogen fertilizer application (120 kg ha-1)
The boxes in the figure indicate the interquartile range, and the bars indicate the maximum and minimum values. Soil layers below 10 cm depth have less ammonium applied, so their nitrification activity does not increase and the effect of sorgoleone does not appear. -
Fig. 4. Gene abundance in bulk (no crop) and rhizosphere soils (0-10 cm layer) of sorghum lines cultivated for 70 days under nitrogen fertilizer application (120 kg ha-1)
amoA: Ammonia monooxygenase α subunit gene. Bars in the figure indicate standard deviations. The same type of letter (x, x′, X, X′) compares the differences of the abundance of each gene, and values with the same letter are not significantly different at p<0.05. The gene abundance below 10 cm showed a similar trend.
- Affiliation
-
Japan International Research Center for Agricultural Sciences Crop, Livestock and Environment Division
- Classification
-
Technical A
- Research project
- Program name
- Term of research
-
FY2019 (FY2016-FY2020)
- Responsible researcher
-
Sarr Papa Saliou ( Crop, Livestock and Environment Division )
ORCID ID0000-0002-4478-4463Ando Yasuo ( Research Strategy Office )
KAKEN Researcher No.: 80353548Nakamura Satoshi ( Crop, Livestock and Environment Division )
ORCID ID0000-0002-0952-5618KAKEN Researcher No.: 00749921Subbarao Guntur Venkata ( Crop, Livestock and Environment Division )
ORCID ID0000-0002-7243-6394KAKEN Researcher No.: 00442723Deshpande Santosh ( International Crops Research Institute for the Semi-Arid Tropics )
- ほか
- Publication, etc.
-
https://doi.org/10.1007/s00374-019-01405-3
Sarr PS et al. (2019) Biology and Fertility of Soils, 56(2):145-166
- Japanese PDF
-
2019_A05_A4_ja.pdf607.67 KB
2019_A05_A3_ja.pdf228.79 KB
- English PDF
-
2019_A05_A4_en.pdf206 KB
2019_A05_A3_en.pdf276.23 KB
- Poster PDF
-
2019_A05_poster_fin.pdf367.71 KB