Biological Nitrification Inhibition - Dual benefit for agriculture and the environment
Description
Most modern agricultural systems are based on large inputs of nitrogen (N) with ammonium (NH4+) being the primary N source. In addition, current crop management practices result in the development of highly nitrifying soil environments. Nitrification results in the transformation of the relatively immobile NH4+ to highly mobile nitrate (NO3-), making inorganic N susceptible to losses through leaching of NO3- and/or gaseous N emissions, initiating a cascade of environmental and health problems. Nitrous oxide (N2O), a powerful greenhouse gas contributes to global warming, produced primarily from nitrification and denitrification processes. Though nitrification is one of the critical processes of the nitrogen cycle, unrestricted and rapid nitrification in agricultural soils can result in major N losses from the plant-soil systems. The low agronomic N-use efficiency (NUE) found in many agricultural systems, is largely the result of these N losses. Most plants have the ability to assimilate both NH4+ and NO3-; therefore nitrification does not need to be a dominant process in the N cycle for efficient N use.
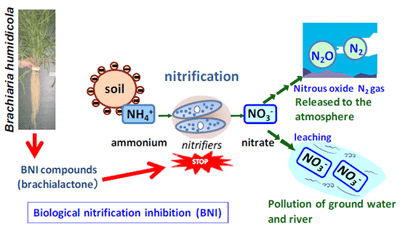
The ability of certain plant species to release organic molecules/compounds from their roots that specifically inhibit the function of nitrifying soil bacteria is a phenomenon termed “biological nitrification inhibition” (BNI). A schematic presentation of the BNI concept along with various processes of the soil N-cycle that are impacted is presented in Fig. 1. We adopted a very sensitive bioassay using a recombinant luminescent Nitrosomonas europaea to detect biological nitrification inhibition (BNI) in plant-soil systems, with the inhibitory activity of roots expressed in allylthiourea units (ATU). Such BNI capacity appears to be relatively widespread among tropical pasture plants, with Brachiaria species showing the highest capacity among the pasture grasses tested.
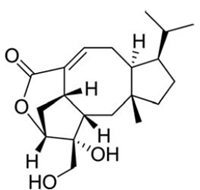
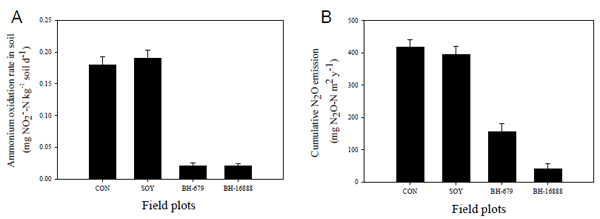
Here we report the discovery of an effective nitrification inhibitor in the root exudates of a tropical grass Brachiaria humidicola (Rendle) Schweick. Named “brachialactone”, this inhibitor is a recently discovered cyclic diterpene with a unique 5-8-5-membered ring system and a g-lactone ring (Fig. 2). It contributed 60-90% of the inhibitory activity released from the roots of this tropical grass. Unlike nitrapyrin (a synthetic nitrification inhibitor), which affects only the ammonia monooxygenase (AMO) pathway, brachialactone appears to block both AMO and hydroxylamine oxidoreductase (HAO) enzymatic pathways in Nitrosomonas. Release of this inhibitor is a regulated plant function, triggered and sustained by the availability of ammonium (NH4+) in the root environment. Brachialactone release is restricted to those roots that are directly exposed to NH4+. Within three years of establishment, Brachiaria pastures have suppressed soil nitrifier populations (determined as amoA genes; ammonia-oxidizing bacteria and ammonia-oxidizing archaea), along with nitrification and nitrous oxide emissions (Fig. 3a,b).
These findings provide the direct evidence for the existence and active regulation of a nitrification inhibitor (or inhibitors) release from tropical pasture root systems. Development of improved forage grasses for low nitrifying pasture-based production systems is possible given the significant variability found for the BNI function within the Brachiaria spp (Fig. 3a,b). A fundamental shift towards NH4+-dominated crop nutrition can thus be achieved by using crops and pastures that have high BNI-capacity or integrating annual crop production with a high BNI-capacity forage component, resulting in low-nitrifying agronomic production systems, benefiting both agriculture and the environment.
Figure, table
-
Fig. 1. Schematic representation where biological nitrification (BNI) interfaces with the nitrogen cycle -
Fig. 2. Chemical structure of brachialactone (PNAS 2009;106:17302-17307). -
Fig. 3. Influence of tropical pasture grass cultivation on soil nitrification and nitrous oxide emissions
(A) Soil ammonium oxidation rate in field plots planted with tropical pasture grasses and soybean
(B) Cumulative N2O emissions from field plots of tropical pasture grasses (monitored monthly over a 3-year period, from September 2004 to November 2007). [CON, control (plant-free) plots; SOY, soybean; BH-679, B. humidicola CIAT 679; BH-16888, B. humidicola CIAT 16888; (PNAS 2009;106:17302-17307)]. -
- Affiliation
-
Japan International Research Center for Agricultural Sciences Biological Resources Division
- Classification
-
Administration A
- Term of research
-
FY2009(FY2006~2010)
- Responsible researcher
-
SUBBARAO Guntur Venkata ( Biological Resources Division )
ISHIKAWA Takayuki ( Crop Production and Environment Division )
NAKAHARA Kazuhiko ( Post-harvest Science and Technology Division )
YOSHIHASHI Tadashi ( Post-harvest Science and Technology Division )
ITO Osamu ( Biological Resources Division )
ONO Hiroshi ( Biological Resources Division )
Kameyama Mayumi ( National Food Research Institute )
KAKEN Researcher No.: 80353994Yoshida Mitsuru ( National Food Research Institute )
KAKEN Researcher No.: 60353992 - ほか
- Publication, etc.
-
Subbarao et al. (2009) Proc. Natl. Acad. Sci. USA. 106(41):17302-17307
- Japanese PDF
-
2009_seikajouhou_A4_ja_Part6.pdf102.6 KB