Identification and characterization of biological nitrification inhibition (BNI) substances in sorghum root
Description
Nitrification, one of several pathways in the soil-N cycle, results in the microbiological conversion of relatively immobile NH4+ into highly mobile NO3-(which is susceptible to losses through leaching [NO3-leaching]), and gaseous N emissions (N2O, NO and N2) by denitrification. The price of nitrogen fertilizer has been rising in recent years. Controlling nitrification through suppression of nitrifier activity is thus critical to improving nitrogen use efficiency (NUE) of agricultural production systems. Suppressing soil nitrification through the release of nitrification inhibitors from plant roots is termed ‘biological nitrification inhibition’ (BNI). This present study aims to characterize BNI function in sorghum, in particular the production of inhibitors, their chemical identity, functionality, and factors regulating their release.
Sorghum roots release two types of nitrification inhibitors: hydrophilic-BNIs and hydrophobic-BNIs. The former were those released into water-based collection medium while the latter are those released by washing the roots for 30s with dichloromethane (DCM), which has high affinity for hydrophobic compounds.
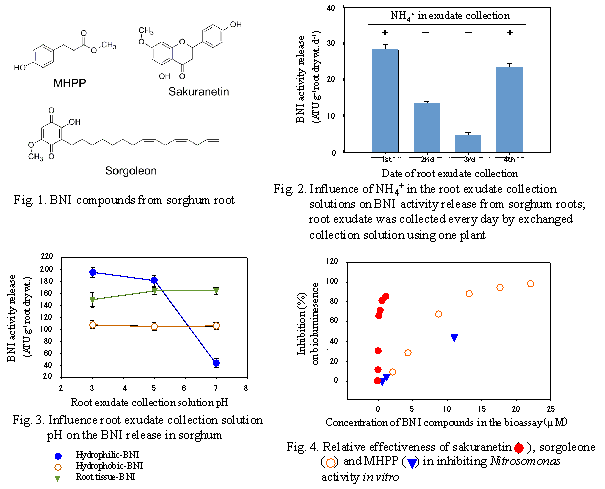
Three nitrification inhibitors -- MHPP (methyl 3-(4-hydroxyphenyl) propionate), sakuranetin (5,4’-dihydroxy-7-methoxyflavanone) (isolated from hydrophilic BNI activity), and sorgoleone (2-hydroxy-5-methoxy-3-[(8’Z,11’Z)-8’,11’,14’-pentadecatriene]-p-benzoquinone) (isolated from hydrophobic BNI activity) -- were isolated from the inhibitory activity released from sorghum roots (Fig. 1). The release of nitrification inhibitors required the presence of NH4+, whose stimulatory effect lasted 24h, in the root environment (Fig. 2). The release of hydrophilic-BNIs declined at rhizosphere pH > 5.0. Nearly 80% of hydrophilic-BNI released was suppressed at pH ≥7.0 (Fig. 3). A bioluminescence assay using recombinant Nitrosomonas europaea was employed to determine BNI activity. The ED80(effective dose for 80% inhibition Nitrosomonas function) for sakuranetin, sorgoleone, and MHPP was 0.6 µM, 12.0µM, and >120 µM, respectively (Fig. 4).
These results are useful as fundamental knowledge towards utilization research of BNI in sorghum. We should clarify the field conditions in which sorghum BNI is the most efficient and investigate the BNI activity of each substance in the soil. We need to establish reliable screening techniques and selection criteria for breeding.
Figure, table
- Affiliation
-
Japan International Research Center for Agricultural Sciences Crop, Livestock and Environment Division
- Classification
-
Administration B
- Research project
- Program name
- Term of research
-
FY 2006- FY 2010
- Responsible researcher
-
Subbarao Guntur Venkata ( Crop, Livestock and Environment Division )
ORCID ID0000-0002-7243-6394KAKEN Researcher No.: 00442723Nakahara Kazuhiko ( Biological Resources and Post-harvest Division )
Zakir H. A. K. M. ( Crop, Livestock and Environment Division )
Ishikawa Takayuki ( Crop, Livestock and Environment Division )
Yoshihashi Tadashi ( Crop, Livestock and Environment Division )
Ono Hiroshi ( National Food Research Institute )
KAKEN Researcher No.: 90353995Yoshida Mitsuru ( National Food Research Institute )
KAKEN Researcher No.: 60353992 - ほか
- Publication, etc.
-
https://doi.org/10.1007/s11104-012-1419-9
Subbarao et al. (2012) Plant Soil
Zakir et al. (2008) New Phytologist, 180:442-451
- Japanese PDF
-
2012_04_A4_ja.pdf33.44 KB
- English PDF
-
2012_04_A4_en.pdf148.5 KB