Development of cellulose-degrading enzyme recycle system for reducing enzyme cost of saccharification
Description
Lignocellulosic plant biomass is difficult to hydrolyze because cellulose is surrounded by a lignin that has covalent associations with hemicellulose, and cellulose has a tightly packed crystalline structure. Thus, the rate-limiting step in biomass degradation is the conversion of cellulose and hemicellulose polymers to sugars. Among cellulolytic microorganisms, Clostridium thermocellum, an anaerobic thermophilic bacterium, is the most potent cellulose degrading bacterium known to produce the cellulosome (2-3.5 MDa). The cellulosome structure of C. thermocellum consists of a large non-catalytic scaffolding protein (scaffoldin) named CipA of 197 kDa that is multi-modular and includes nine cohesins, and a family III cellulose binding module (CBM). The catalytic units are non-covalently attached to scaffoldin via the type I interaction between dockerin domains borne by the catalytic units with the cohesins on the scaffoldin.
Recently, to isolate microorganisms that possess effective cellulose-degrading ability, new thermophilic cellulolytic strains were screened from agriculture residues in Thailand using microcrystalline cellulose as a carbon source. We isolated a new strain, C. thermocellum S14, which has higher cellulose-degrading ability than several type strains (1).
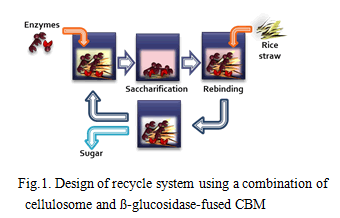
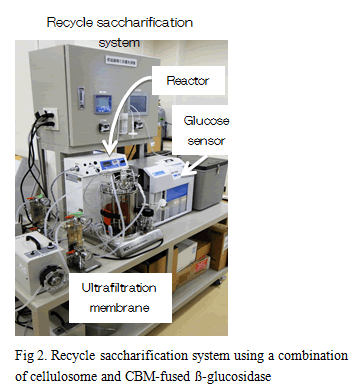
When rice straw treated by soaking in aqueous ammonia was hydrolyzed by the combination of ß-glucosidase from Thermoanaerobacter brockii with the cellulosome from C. thermocellum S14, approximately 91% of glucan existing in the rice straw was hydrolyzed (2). On the other hand, enzyme recycling is desired to reduce costs of saccharification process (Fig. 1). In order to recycle the combination, CBM from CipA was fused to the ß-glucosidase. When recycling tests were carried out against crystalline cellulose and ammonia-treated rice straw, combination of cellulosome and ß-glucosidase-fused CBM could recycle at least 5 and 4 rounds, respectively, consistent with high saccharification rates. Based on these results, a recycle saccharification reactor system that can recover saccharified solution through an ultrafiltration membrane using a combination of the cellulosome and ß-glucosidase-fused CBM was developed (Fig. 2). These results indicated that the combination of cellulosomes and β-glucosidase from thermophilic anaerobic microorganisms has great potential as an effective lignocellulose degradation system.
Figure, table
- Affiliation
-
Japan International Research Center for Agricultural Sciences Biological Resources and Post-harvest Division
- Classification
-
Research A
- Research project
- Program name
- Term of research
-
FY 2011(FY 2006-FY 2011)
- Responsible researcher
-
Kosugi Akihiko ( Biological Resources and Post-harvest Division )
Waeonukul Rattiya ( King Mongkut's Institute of Technology Ladkrabang )
Tachaapaikoon Chakrit ( King Mongkut's Institute of Technology Ladkrabang )
ORCID ID0000-0001-9795-2558Mori Takashi ( Biological Resources and Post-harvest Division )
- ほか
- Publication, etc.
-
Tachaapaikoon C, Kosugi A, Pason P, Waeonukul R, Ratanakhanokchai K, Kyu KL, Arai T, Murata Y, Mori Y. (2012) Isolation and characterization of a new cellulosome-producing Clostridium thermocellum strain. Biodegradation. 23(1):57-68.
Waeonukul R, Kosugi A, Tachaapaikoon C, Pason P, Ratanakhanokchai K, Prawitwong P, Deng L, Saito M, Mori Y. (2012) Efficient saccharification of ammonia soaked rice straw by combination of Clostridium thermocellum cellulosome and Thermoanaerobacter brockii β-glucosidase. Bioresource Technology 17:352–357
Kosugi, et al. (2010) "Method for reutilizing enzyme". JP2011044541A
- Japanese PDF
-
2011_13_A4_ja.pdf139.11 KB
- English PDF
-
2011_13_A4_en.pdf199 KB