Development of innovative enzyme technology for cellulosic biomass degradation
Description
Among the cellulosome-producing microorganisms, Clostridium thermocellum, an anaerobic, thermophilic and spore-forming bacterium, has the most potent cellulose-degrading ability, and thus it attracted wide attention as a source of cellulolytic enzymes to hydrolyze lignocelluloses into glucose and other sugars which then can be converted into useful substances such as ethanol. The cellulosome (2-3.5 MDa) of C. thermocellum consists of a large (197 kDa), non-catalytic, multi-modular scaffolding protein named CipA that includes nine cohesins, four hydrophilic modules and a family III cellulose-binding module (CBM). The catalytic units are non-covalently attached to the scaffoldin via high affinity-type I interactions between the dockerin domains of the catalytic units with the cohesins on the scaffoldin. We isolated the C. thermocellum strain S14 (NITE P-627), which was originally isolated from bagasse paper sludge in Thailand, and which produces cellulosome with strong cellulolytic activity. To bring out the cellulolytic abilities of the cellulosome, it is necessary to eliminate inhibition against it by the major end product, cellobiose. Combining recombinant β-glucosidase from Thermoanaerobacter with the cellulosome from C. thermocellum S14, accumulated cellobiose was hydrolyzed thereby resulting in significant enhancement of the microcrystalline cellulose degradation. On the other hand, when rice straw treated by soaking with aqueous-ammonia (28%) solution for 1 week at 60°C was hydrolyzed by a combination of 10 units of β-glucosidase and cellulosome loading of 2 mg protein/g glucan, the maximum saccharification rate was achieved up to 91%. Hydrolysates from rice straw through the combination of β-glucosidase and cellulosome also could be fermented to ethanol by using yeast, Saccharomyces cerevisiae, without any inhibitions up to a theoretical yield of approximately 95%. These results strongly suggest that the combination of β-glucosidase and cellulosome has great potential as a new effective lignocellulose degradation system.
Figure, table
-
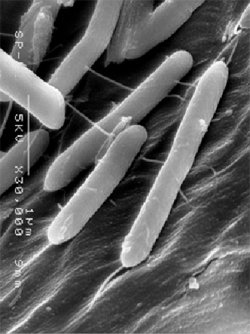
Fig.1. SEM picture of isolated C. thermocellum S14 -
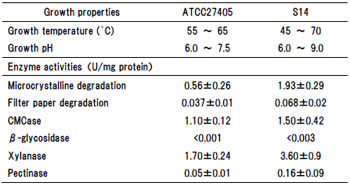
Table 1. Growth and enzyme properties of C. thermocellum S14 strain.
-
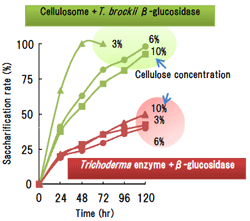
Fig.2.
Saccharification of microcrystalline cellulose using the enzyme combination of cellulosome and T. brockii β-glucosidase. β-glucosidase of Aspergillus niger was used in case of fungi cellulase. The enzyme concentration used is 1 mg per 1 g for 3% and 6% of cellulose substrate and 2 mg per 1 g at 10%. -
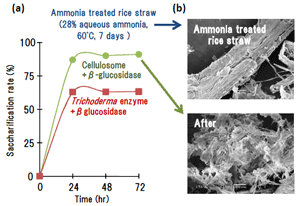
Fig. 3.
Saccharification of ammonia-treated rice straw using the enzyme combination of cellulosome and T. brockii β-glucosidase.
(a) Saccharification profile of ammonia-treated rice straw;
(b) SEM pictures after saccharification.
- Affiliation
-
Japan International Research Center for Agricultural Sciences Post-harvest Science and Technology Division
- Classification
-
Research B
- Term of research
-
FY2006~2010
- Responsible researcher
-
KOSUGI Akihiko ( Post-harvest Science and Technology Division )
Tachaapaikoon Chakrit ( King Mongkut's University of Technology Thonburi )
ORCID ID0000-0001-9795-2558Waeonukul Rattiya ( King Mongkut's University of Technology Thonburi )
MORI Yutaka ( Post-harvest Science and Technology Division )
- ほか
- Publication, etc.
-
Kosugi. et al. Japanese Patent Application No. 2008-222957.
Kosugi. et al. Japanese Patent Application No. 2009-277079. "Method for producing cellulase using clostridium microorganism and method for culturing and proliferating clostridium microorganism".
- Japanese PDF
-
2010_seikajouhou_A4_ja_Part13.pdf301.09 KB