Germ cell cryopreservation to conserve genetic diversity in shrimps
Description
Establishing sustainable aquaculture requires the development of techniques to prevent genetic deterioration of aquaculture populations and to produce superior strains such as disease-resistant strains. For this purpose, it is extremely important to preserve existing genetic diversity. In recent years, germ cell cryopreservation techniques and germ cell transplantation techniques for producing sperm, eggs, and larvae derived from cryopreserved germ cells have been developed for several fish species and are the only effective methods for preserving all genetic information in fish in which egg cryopreservation has not been available. However, such technology has not yet been developed for crustaceans, which are important species in fisheries. The only way to preserve genetic diversity of crustacean species is to keep a huge number of live individuals in aquariums or cages. To address this issue, this study will develop the first germ cell cryopreservation technique in crustacean for two species of prawns, the black tiger shrimp (Penaeus monodon) and banana shrimp (Fenneropenaeus merguiensis), both of which belong to Penaeidae.
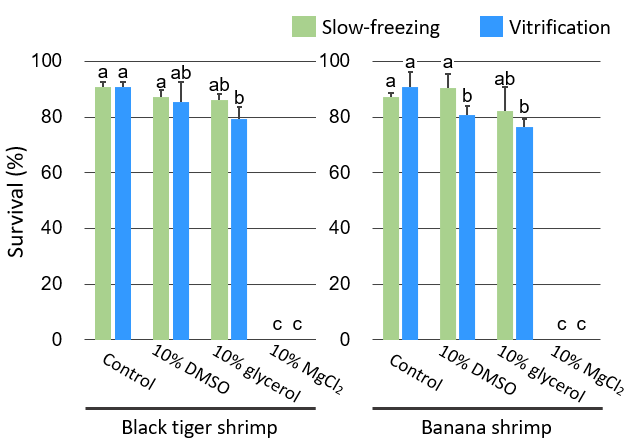
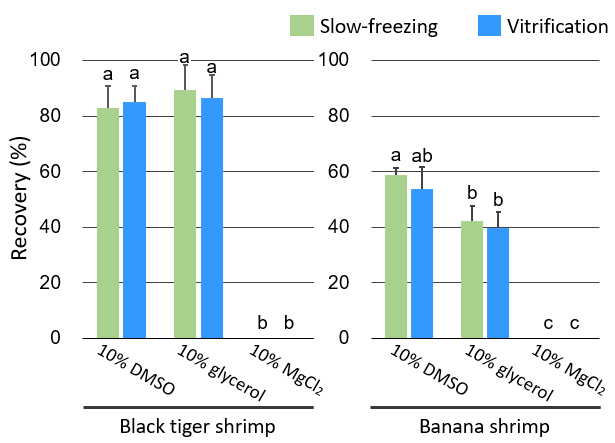
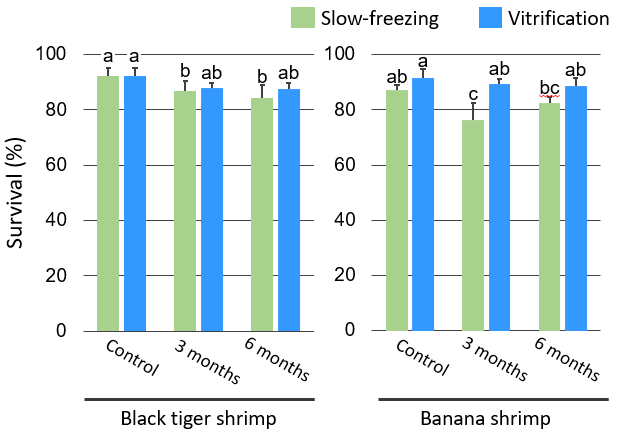
In this study, we compared three commonly used cryoprotectants — dimethyl sulfoxide (DMSO), glycerol, and magnesium chloride (MgCl2) — and found that 10% glycerol or 10% DMSO resulted in higher germ cell viability after cryopreservation in both species (Fig. 1). In terms of recovery rates, 10% glycerol and 10% DMSO resulted in higher recovery rates than other cryoprotectants in black tiger shrimp and banana shrimp, respectively (Fig. 2). Considering the survival and recovery rates together, it was concluded that 10% glycerol and 10% DMSO were suitable cryoprotectants for black tiger shrimp and banana shrimp, respectively. Furthermore, for both species, the vitrification method was found to be more suitable for long-term preservation than the slow-freezing method (Fig. 3).
Based on this study, the following are expected: 1) Since germ cells can be cryopreserved in liquid nitrogen storage containers, this technique will enable the preservation of genetic diversity of the black tiger shrimp and banana shrimp semipermanently without genetic deterioration and with less space and labor than rearing of live individuals; 2) cryopreservation of germ cells makes it possible to prevent genetic deterioration of aquaculture populations and to secure genetic breeding material which will be used in future breeding programs to produce superior strains before genetic diversity is lost, which can lead to sustainable shrimp aquaculture technology; and 3) based on this study, it is expected that germ cell cryopreservation techniques can be developed for other species of prawns, including many species with high economic value, to conserve the existing genetic diversity of these species. In addition to this study, if germ cell transplantation techniques are developed in crustaceans, it will also be possible to regenerate individuals from frozen germ cells.
Figure, table
-
Fig. 1. Survival rates of the germ cells
Different letters indicate significant differences (p < 0.05).
Slow-freezing method is freezing at a rate of –1 ºC/min.
The vitrification method is a rapid freezing method using liquid nitrogen after dehydration and complete replacement of intracellular water with a cryoprotectant. -
Fig. 2. Recovery rates of the germ cells
Recovery rate (%) = the number of live germ cells obtained after freezing and thawing x 100 / the number of live germ cells before freezing and thawing. Different letters indicate significant differences (p < 0.05). -
Fig. 3. Survival rates of the germ cells after long-term cryopreservation
Different letters indicate significant differences (p < 0.05).Figures reprinted/modified with permission from Rakbanjong et al. (2021).
- Classification
-
Research
- Program name
- Research Project
-
SATREPS: Utilization of Thailand Local Genetic Resources to Develop Novel Farmed Fish for Global Market
- Term of research
-
FY 2019–2023
- Responsible researcher
-
Rakbanjong Natthida ( Prince of Songkla University )
Okutsu Tomoyuki ( Fisheries Division )
KAKEN Researcher No.: 40456322Chotigeat Wilaiwan ( Prince of Songkla University )
Songnui Anida ( Department of Fisheries, Thailand )
Wonglapsuwan Monwadee ( Prince of Songkla University )
- ほか
- Publication, etc.
-
Rakbanjong et al. (2021) Marine Biotechnology 23: 590−601https://doi.org/10.1007/s10126-021-10048-1
- Japanese PDF
-
2021_B08_ja.pdf369.29 KB
- English PDF
-
2021_B08_en.pdf298.06 KB
- Poster PDF
-
2021_B08_poster.pdf318.9 KB
* Affiliation at the time of implementation of the study.