A facultatively anaerobic bacterium, Paenibacillus curdlanolyticus B-6, produces a novel xylanolytic multienzyme complex.
Description
Plant biomass contains a complex mixture of polysaccharides such as cellulose, hemicellulose (xylan and galactomannan), pectic substances (galacturonan and arabinogalactan) and other polysaccharides (e.g. type II arabinogalactan and fuco-xyloglucan). The hemicellulose and pectin polysaccharides, as well as the aromatic polymer lignin, interact with the cellulose fibrils, creating a rigid structure strengthening the plant cell wall. Therefore, complete and rapid hydrolysis of these polysaccharides requires not only b-1,4-glycosidic chain-cleaving enzymes such as endo-b-1,4-glucanase, cellobiohydrolase, and b-glycosidase, but also the cooperation of many enzymes such as xylanolytic enzymes and side chain-cleaving enzymes such as b-1,4-xylanase and a-L-arabinofuranosidase.
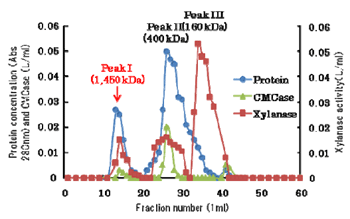
A facultatively anaerobic bacterium Paenibacillus curdlanolyticus B-6, isolated from an anaerobic digester fed with pineapple wastes is a true cellulolytic/xylanolytic organism, as it could grow on xylan, microcrystalline cellulose and α-cellulose as a sole source of carbon under aerobic conditions. P. curdlanolyticus B-6 grown on xylan under aerobic conditions produced two extracellular multienzyme complexes with high molecular weights estimated at 1,450 kDa and 400 kDa. To characterize the multienzyme complexes, we purified the complexes from culture supernatants using four kinds of chromatography.
The purified multienzyme complex was composed of a 280 kDa protein with xylanase activity, a 260 kDa protein that was a truncated form on the C-terminal side of the 280 kDa protein, two xylanases of 40 kDa and 48 kDa, and 60 kDa and 65 kDa proteins having both xylanase and CMCase activities. Cloning of the 40 kDa major xylanase subunit named Xyn11A revealed that Xyn11A contained two functional domains which belonged to glycosyl hydrolase family-11 and to carbohydrate binding module family-36, respectively, and a glycine and asparagine-rich linker. However, an amino acid sequence similar to a dockerin domain, which is crucial to cellulosome assembly, was not found in Xyn11A. These results suggest that the multienzyme complex produced by P. curdlanolyticus B-6 could have assembled through a mechanism distinct from the cohesin-dockerin interactions known in cellulosomes.
Figure, table
-
Fig 1. Gel filtration chromatography using culture supernatant of P. curdlanolyticus B-6 -
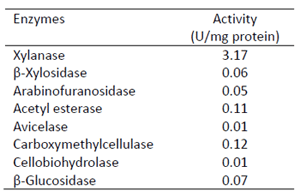
Table 1. Enzymatic activities of multienzyme complex protein with 1,450kDa from gel filtration
-
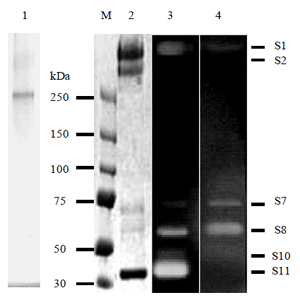
Fig 2. Xylanosome of P. curdlanolyticus B-6
M; Molecular marker: Lane 1, Native-PAGE: Lane 2, SDS-PAGE; Lane 3, Xylanase active staining analysis, Lane 4,endoglucanase active staining analysis. The S1,S2, S7, S8, S10, and S11 indicated subunits with enzymatic activities. -
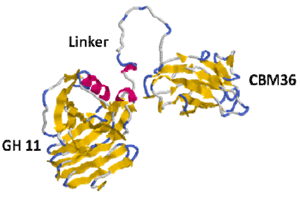
Fig 3. Protein structure model of XynA (S11) of xylanosome of P. curdlanolyticus B-6
Protein structure of Xyn11A revealed glycoside hydrolase family-11 (GH11), linker sequence (Linker) and carbohydrate binding module family-36 (CBM36) from gene sequence. Yellow and red colors indicate β-sheet structure and α-helix structure, respectively.
- Affiliation
-
Japan International Research Center for Agricultural Sciences Post-harvest Science and Technology Division
- Classification
-
Administration B
- Term of research
-
FY2009(FY2006~2010)
- Responsible researcher
-
KOSUGI Akihiko ( Post-harvest Science and Technology Division )
Pason Patthra ( King Mongkut's University of Technology Thonburi )
MORI Yutaka ( Post-harvest Science and Technology Division )
- ほか
- Publication, etc.
-
Pason et al. (2009) Appl Microbiol Biotechnol. 85:573-580
- Japanese PDF
-
2009_seikajouhou_A4_ja_Part9.pdf372.57 KB