An osmosensor as a molecular tool for the genetic improvement of drought-tolerant crops
Description
[Objectives]
In marginal or arid lands in developing countries, environmental factors such as drought, high salinity, high temperature, and flooding are serious problems that lead to instable crop productivity. To address this problem, researchers had developed stress-tolerant plants by transferring a gene encoding protective proteins or enzymes involved in stress tolerance from various organisms. These past efforts had limited success, however, due to the genetic complexity of stress responses and adaptation. Prior shortcomings have indicated that introducing a large number of genes into a plant is necessary to confer fully improved stress tolerance. Thus, we need to manipulate a regulatory gene that controls the quantities and timing of the numerous effecter molecules described above. Under drought conditions, a change in cellular osmotic pressure caused by water loss triggers various intracellular responses. The first step in this event is perception of osmotic changes by a sensor or receptor protein(s) at plasmamembrane. Therefore, we decided to target the osmosensor as the regulatory gene to be engineered.
[Results]
ATHK1 contains two hydrophobic transmembrane regions adjacent to a putative extracellular domain in the Nterminal half, suggesting functional similarity with the yeast osmosensor SLN1. Overexpression of the ATHK1 cDNA suppressed the lethality of a yeast sln1 mutant. Moreover, introduction of the ATHK1 cDNA into an yeast mutant lacking both osmosensors, SLN1 and SHO1, allowed normal growth and activation of the HOG1 MAPK cascade under the high osmolarity condition, suggesting that the ATHK1 activity changed to inactive state from active state in response to increases in external osmolarity. Thus, we demonstrated, by analyzing both sensing (input) and catalytic (output) activities of ATHK1 in vivo using the yeast osmosensing-defective mutants, that ATHK1 has an ability to sense and transduce a signal of external osmolarity to the downstream targets. In order to exammine the function of ATHK1 in planta, we initially screened dominant-negative ATHK1 mutants that inhibit the activity of the wild-type ATHK1 and isolated six candidates (ATHK1-1 to 6). We then generated transgenic Arabidopsis plants overexpressing the dominant-negative ATHK1 cDNAs. cDNA microarry and following northern blot analyses indicated that a number of stress-inducible genes are constitutively expressed in the dominant-negative ATHK1 overexpressors under the unstressed condition (Fig. 1). Moreover, the dominantnegative ATHK1 overexpressors were tolerant to dehydration and high salinity stresses than wild-type plants (Fig. 2). These results, together with yeast genetic analysis, suggest that ATHK1 is an osmosensor in Arabidopsis. This is the first evidence that a plant histidine kinase acts as an osmosensor. The ATHK1 gene could be one of the most useful molecular tools or biological resources for the genetic improvement of drought-tolerant crops.
Figure, table
-
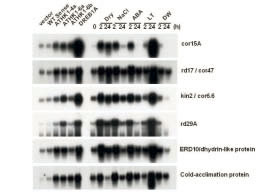
Fig. 1. Northern blot analysis of stress-inducible genes.
Expression levels of a number of stressinducible genes were higher in all of the dominant negative ATHK1 (1-4a, 1-6a, 1-6b) and DREB1A overexpressors (DREB1A) than those in the control (vector) and wild-type ATHK1 overexpressors (WT sense) under unstressed conditions. These genes were also induced by dehydration stress, high osmolarity (NaCl), absicic acid (ABA), low temperature (LT) or water (DW) in wild-type Arabidopsis plants. -
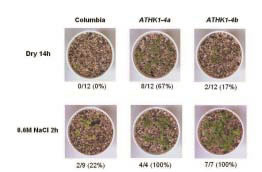
Fig. 2. Drought and high salinity tolerance of the dominant-negative ATHK1 overexpressors.
3-week-old plants were dehydrated for 14 hours on paper and re-hydrated for 2 days with water. The plants were then transferred to a pot for 5 days. 67% and 17%, respectively, of the dominant-negative ATHK1 overexpressors survived, but all wild-type plants died under the same conditions. 3-week-old plants were exposed to 600 mM NaCl solution for 2 hours and then grown in a pot for 5 days. The salt tolerance of the dominantnegative ATHK1 overexpressers (100% survival, respectively) was much stronger than that of the wild-type plants (22% survival).
- Affiliation
-
Japan International Research Center for Agricultural Sciences Biological Resources Division
- Classification
-
Technical A
- Term of research
-
FY2002 (FY1998-2002)
- Responsible researcher
-
URAO Takeshi ( Biological Resources Division )
YAMAGUCHI-SHINOZAKI Kazuko ( Biological Resources Division )
- ほか
- Publication, etc.
-
Urao, T., Yakubov, B., Satoh, R., Yamaguchi-Shinozaki, K., Seki, M., Hirayama, T. and Shinozaki, K. (1999): A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell, 11, 1743-1754.
Urao, T., Yamaguchi-Shinozaki, K. and Shinozaki, K. (2000): Two-component system in plant signal transduction. Trends in Plant Science, 5, 67-74.
Urao, T., Yamaguchi-Shinozaki, K. and Shinozaki, K. (2001): Plant histidine kinases: An emerging picture of two-component signal transduction in hormone and environmental responses. Science's STKE, Review, 21.
- Japanese PDF
-
2002_04_A3_ja.pdf887.55 KB
- English PDF
-
2002_04_A4_en.pdf60.05 KB