Sorgoleone release determines the hydrophobic-BNI capacity in sorghum root systems
Description
Nitrification and denitrification are the two most important processes that contribute to greenhouse gas emissions and the inefficient use of nitrogen. Suppressing soil nitrification through the release of nitrification inhibitors from roots is a plant function, termed ‘Biological Nitrification Inhibition (BNI)’. Sorghum releases two categories of nitrification inhibitors from roots: hydrophilic BNIs and hydrophobic BNIs. Our earlier published work on sorghum mostly focused on characterizing hydrophilic BNI release. Here we report the characterization of hydrophobic-BNI release in sorghum. The functional role and contribution of sorgoleone release to hydrophobic-BNI function and the existence of genotypic variability for sorgoleone release is the focus of this investigation. Three sorghum genotypes (Hybridsorgo, IS 41245 and GDLP 35-5-5-3) were evaluated for their capacity to release sorgoleone in hydroponic, in soil culture, and under field environments. Sorgoleone released from roots is measured using a high performance liquid chromatograph (HPLC) and BNI activity is determined using a luminescent recombinant Nitrosomonas europaea assay.
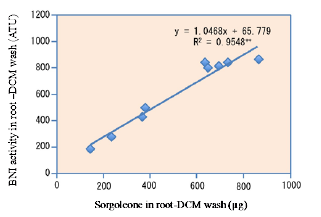
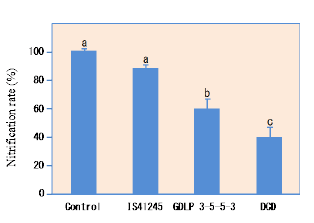
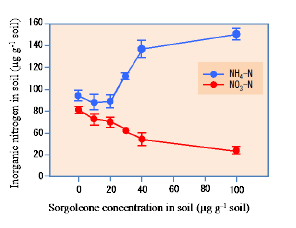
Sorgoleone was found to be the dominant and major component of hydrophobic-BNI activity released from sorghum roots, and there were significant genotypic differences for sorgoleone release (Fig. 1). Sorgoleone release and BNI-activity release in sorghum roots are closely associated, i.e., 1 μg of sorgoleone released is equivalent to 1 ATU activity in the bioassay (Fig. 2). Sorgoleone genotypes release varying quantities of sorgoleone. GDLP 34-5-5-3 and Hybridsorgo have higher capacity for both sorgoleone release and BNI activity than IS41245. In soil culture, GDLP 34-5-5-3 released significantly higher quantities of sorgoleone into the rhizosphere, had higher BNI activity, and suppressed soil nitrification better than IS41245 (Fig. 3). Purified sorgoleone inhibited Nitrosomonas activity in the bioassay; when amended to soil, sorgoleone suppressed nitrification, improved NH4+ availability, and reduced NO3- formation in soils during a 60-day incubation study (Fig. 4). These results demonstrate genetic differences for sorgoleone release and its functional link to hydrophobic-BNI release and BNI capacity in sorghum.
Sorgoleone release contributes significantly to BNI capacity in sorghum. The significant genetic differences for sorgoleone release from sorghum roots suggest that there is potential for genetic improvement to improve sorgoleone release and BNI capacity in sorghum. Higher BNI capacity is critical to the development of low-nitrifying sorghum production systems and the results presented here suggest the feasibility of this approach.
Figure, table
-
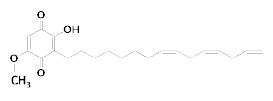
Fig. 1. Chemical structural formula of sorgoleone
-
Fig. 2. The relationship between total sorgoleone concentration (μg) and BNI activity (ATU) in root–DCM wash of three sorghum genotypes
-
Fig. 3. Nitrification rate at 30-day incubation period along with NH4+ inoculation of rhizosphere soil collected from two sorghum genotypes (IS1245 and GDLP 34-5-5-3) grown up to heading stage in potted soil. Control pots were included with bare soil without plants but handled the same way like pots with plants. As positive control, soils taken from control treatments were also incubated with DCD addition at 25 ppm (a known synthetic inhibitor) as a reference.
-
Fig. 4. Concentration of inorganic N (NO3− and NH4+) in soil samples incubated after adding different concentrations of sorgoleone (0, 10, 20, 30, 40, and 100 μg g−1 soil) for 60 days
- Affiliation
-
Japan International Research Center for Agricultural Sciences Crop, Livestock and Environment Division
- Classification
-
Administration B
- Research project
- Program name
- Term of research
-
FY 2011-FY2013 (FY 2011-FY2015)
- Responsible researcher
-
Tesfamariam T. ( Crop, Livestock and Environment Division )
Yoshinaga Hiroki ( Crop, Livestock and Environment Division )
Deshpande Santosh ( International Crops Research Institute for the Semi-Arid Tropics )
Srinivasa Rao P. ( International Crops Research Institute for the Semi-Arid Tropics )
Sahrawat Kanwar L. ( International Crops Research Institute for the Semi-Arid Tropics )
Ando Yasuo ( Crop, Livestock and Environment Division )
KAKEN Researcher No.: 80353548Nakahara Kazuhiko ( Biological Resources and Post-harvest Division )
Hash C. T. ( Crop, Livestock and Environment Division )
Subbarao Guntur Venkata ( Crop, Livestock and Environment Division )
ORCID ID0000-0002-7243-6394KAKEN Researcher No.: 00442723 - ほか
- Publication, etc.
-
https://doi.org/10.1007/s11104-014-2075-z
Tesfamariam, T. et al. (2014) Plant and Soil, 379: 325-335
- Japanese PDF
-
2014_A07_A3_ja.pdf329.49 KB
2014_A07_A4_ja.pdf243.42 KB
- English PDF
-
2014_A07_A3_en.pdf123.97 KB
2014_A07_A4_en.pdf127.86 KB
- Poster PDF
-
2014_A07_poster.pdf378.09 KB