Analysis of the secretion mechanism of the biological nitrification inhibitors from sorghum root
Description
The ability to suppress soil nitrification by releasing nitrification inhibitors from plant root systems is termed 'biological nitrification inhibition' (BNI). We have reported earlier that sorghum roots release higher BNI-activity when grown with NH4++, but not with NO3- as N source. Also, for BNI release, rhizosphere pH of <5.0 is needed; beyond this, a negative effect on BNI release was observed with nearly 80% loss of BNI activity at pH >7.0. This study is aimed at understanding the inter-functional relationships associated with NH4++ uptake, rhizosphere-pH and plasma membrane H+-ATPase (PM H+-ATPase) activity in regulating the release of biological nitrification inhibitors (BNIs).
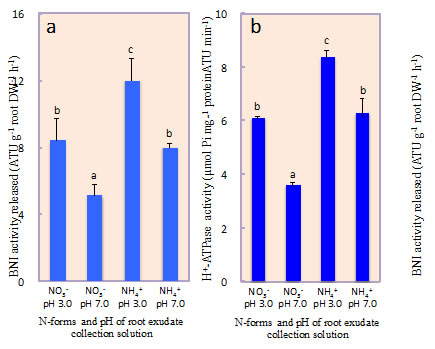
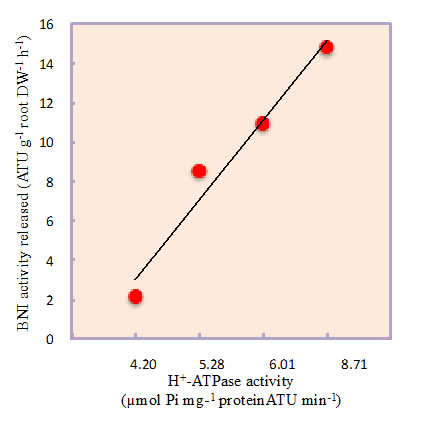
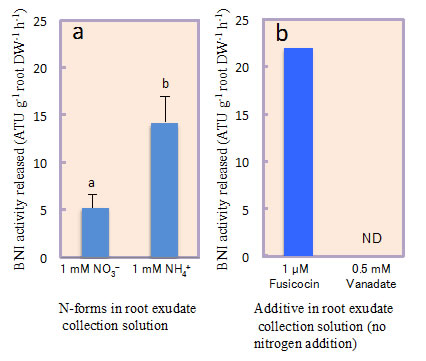
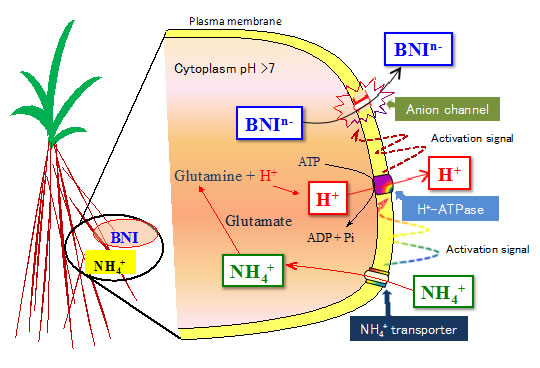
Sorghum was grown hydroponically and root exudate was collected from intact plants using a pH-stat system to separate the secondary acidification effects from NH4++ uptake on BNIs release. A recombinant luminescent Nitrosomonas europaea bioassay was used to determine BNI-activity. Root plasma membrane was isolated using a two-phase partitioning system. Hydrolytic H+-ATPase activity was determined. Split-root system is used to understand the localized responses to NH4++, H+-ATPase-stimulator (fusicoccin) or H+-ATPase-inhibitor (vanadate) on BNI release by sorghum. The results presented suggest that the presence of NH4++ in the rhizosphere stimulated the expression of H+-ATPase activity and enhanced the release of BNIs from sorghum roots compared to NO3- (Fig. 1a, b). NH4++ levels (in rhizosphere) positively influenced BNIs release and root H+-ATPase activity in the concentration range of 0 - 1.0 mM, indicating a close relationship between BNIs release and root H+-ATPase activity with a possible involvement of carrier-mediated transport for the release of BNIs in sorghum (Fig. 2). Split-root system studies show that part of the root system exposed to NH4++ released substantially higher levels of BNI activity than the root system that was exposed to NO3-; similarly, part of the root system which was exposed to fusicoccin, stimulated BNI release, whereas, the part of the root system exposed to vanadate suppressed BNI release (Fig. 3a, b). These results indicate that NH4++ uptake, PM H+-ATPase activity, and rhizosphere acidification are functionally inter-connected with BNI release in sorghum and a hypothesis is proposed (Fig. 4).
Such knowledge is critical to gain insights into why BNI function is likely to be more effective in light-textured and mildly acidic soils [such as Alfisols (of SAT regions), Ultisols (predominant in South America) or Sandy-loams (of West-African SAT region)] compared to heavy-textured soil types such as Vertisols.
Figure, table
-
Fig. 1. Influence of N-forms (i.e. 1 mM N as NH4++ vs. NO3-) and root exudate collection solution pH (solution pH 3.0 vs. 7.0) on biological nitrification inhibition (BNI) release and root plasma membrane (PM) H+-ATPase in sorghum grown hydroponically for 14 days with NH4++ or NO3- as N source.
-
Fig. 2. The relationship between BNI release from sorghum roots and root PM H+-ATPase activity at various concentrations of NH4++ (0 to 1.0 mM) in the root exudate collection solutions.
-
Fig. 3. Influence of N-forms (1 mM N as NH4++ vs. NO3-) and H+-ATPase stimulator, fusicoccin (1 µM) or H+-ATPase inhibitor, vanadate (0.5 mM) on BNI release in sorghum in a split-root system setup.
-
Fig. 4. Hypothesis on the transport of biological nitrification inhibitors (BNIs), driven by plasma membrane H+-ATPase, associated with NH4++ uptake and assimilation in sorghum.
- Affiliation
-
Japan International Research Center for Agricultural Sciences Crop, Livestock and Environment Division
- Classification
-
Administration B
- Research project
- Program name
- Term of research
-
FY 2009-FY 2010 (FY 2006- FY 2010)
- Responsible researcher
-
Zhu Y. ( Nanjing Agricultural University )
Zeng H. ( Nanjing Agricultural University )
Shen Q. ( Nanjing Agricultural University )
Ishikawa Takayuki ( Crop, Livestock and Environment Division )
Subbarao Guntur Venkata ( Crop, Livestock and Environment Division )
ORCID ID0000-0002-7243-6394KAKEN Researcher No.: 00442723 - ほか
- Publication, etc.
-
https://doi.org/DOI: 10.1007/s11104-012-1151-5
Zhu, Y. et al. (2012) Plant and Soil, 358: 131-141
- Japanese PDF
-
2013_A07_A3_ja.pdf1.39 MB
2013_A07_A4_ja.pdf1.66 MB
- English PDF
-
2013_A07_A3_en.pdf188.49 KB
2013_A07_A4_en.pdf154.74 KB
- Poster PDF
-
2013_A07_poster.pdf337.96 KB